check_circle_outlineNon-pathogenic
check_circle_outlineNon-GMO
for your research and development
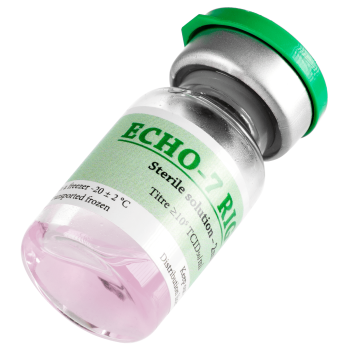
ECHO-7 RIGVIR®
check_circle_outlineGMP quality
check_circle_outline3rd party tested
ECHO-7 RIGVIR® - DESCRIPTION
ECHO-7 RIGVIR® contains a non-genetically modified, non-pathogenic, non-toxic virus. It belongs to the Picornaviridae family, Enterovirus genus, Enteric Cytopathogenic Human Orphan group, type 7 (ECHO-7) and is adapted to cancer cells.
Ingredients: ECHO-7 Rigvir, sodium chloride, sterile water, ECHO-7 proteins, minerals, amino acids, vitamins.
Titre ≥105 TCID50/ml.
One 6 ml vial contains 2 ml solution.
Solution is colourless or slightly pink.
Expiration date 30 months.
Warning – keep out of reach and sight of children.
This product is not a prescription medicine and is intended for science and industry purposes.
Store in a freezer -20 ± 2°C (-0.4°F to -7.6°F). To be transported frozen.
Best before – please see packaging.
Delivery within approximately 10 days from receipt of order. Upon receiving your order, make sure the packaging is not damaged and that the product is frozen.
Shipping costs
EU – 450 EUR (530 USD)
UK – 450 EUR (530 USD)
USA – 650 EUR (765 USD)
* Indicated prices are for shipments up to 100 vials, please contact us for larger orders.
** Delivery to other regions is organized based on individual requests.
You can reach out to us via the contact form located at the bottom of the page.
If, upon receipt of your order, it is found that the packaging is damaged or the product is defrosted, then within two weeks the product is replaced, or you get a refund.
International awards and received grants

In 2016, RIGVIR® project was recognised as a European Innovation and received the Horizon 2020 grant for the commercialization of RIGVIR® within the EU. As a result, permission from the European Medicines Agency to start Phase 2 studies was received.

In 2017, RIGVIR® project was awarded the WIPO IP Enterprise Trophy Award, which emphasises the excellence of protection of Intellectual property.

In 2017, RIGVIR® project was awarded two Seal of Excellence Awards from the European Commission, which certifies the high quality of the project.

In 2019, RIGVIR® received a grant of 360,000 EUR from the European Regional Development Fund (ERDF) to conduct a Rigvir GLP toxicity study.
Safety
Based on study results, virus Rigvir showed high tolerability and did not induce any severe adverse event even at the highest dose tested.
Quality
ECHO-7 RIGVIR® is continuously kept in high-quality condition, paying particular attention to sterility and virus stability (or virus titre (a.k.a. potency)).
Third party tested
Quality and stability tests are regularly carried out in an independent laboratory.
Manufacturing
Manufactured in European Union GMP certified facilities.
Monitoring
Monitoring of product quality is carried out during and after manufacturing, and after the product has entered the market, by regular testing of reserve stocks.
Logistics
Logistics is done in cooperation with international delivery companies that are able to guarantee the cold regime specified by the manufacturer.
Studies
Rigvir GLP toxicity study, performed by Charles River Laboratories UK:
Based on the results, the no-observed-adverse-effect level (NOAEL) was considered to be the highest dose tested.
Oncolytic virus preclinical toxicology studies, Journal of Applied Toxicology:
The article concludes that oncolytic viruses have been shown to have low toxicity and high tolerability in preclinical toxicology studies.
More studies and articles:
Legal announcement
ECHO-7 virus Rigvir exclusive distribution license holder, product buyer and distributor – AramaPharm, Ltd (Estonia). Licensor – Rigvir Group.
ECHO-7 Rigvir is intended for science and industry.
Inventor – Institute of Microbiology and Virology, prof. Aina Muceniece.
Patent and trademark protected internationally.